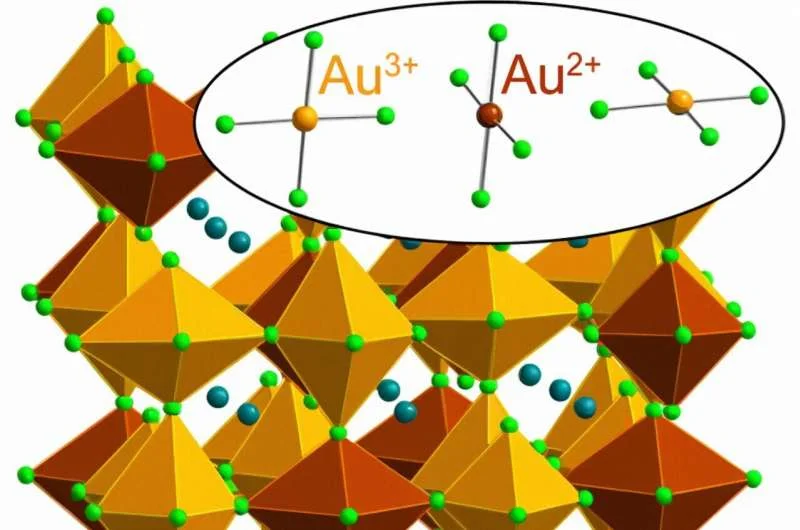
Stanford researchers have found for the first time a way to create and stabilize an extremely rare form of gold that has lost its two negatively charged electrons, known as Au2+. The material that stabilizes this elusive version of the precious element is halide perovskite, a class of crystalline materials that holds great promise for a variety of applications, including more efficient solar cells, light sources and electronic components.
Surprisingly, Au2+ perovskite can also be produced quickly and easily using readily available materials at room temperature.
“The fact that we were able to synthesize a stable material containing Au2+ was a real surprise; I didn’t even believe it at first,” said Hemamala Karunadasa, assistant professor of chemistry at the Stanford School of Humanities and senior author of the study. . The study was published Aug. 28 in the journal Nature Chemistry. “Creating a first-of-its-kind Au2+ perovskite is an exciting endeavor. The gold atoms in the perovskite are very similar to copper atoms in high-temperature superconductors, and heavy atoms with unpaired electrons, such as Au2+, show cold magnetic effects not previously seen in lighter atoms.”
“Halide perovskites have really interesting properties for many everyday applications, so we wanted to expand on this family of materials,” said study lead author Kurt Lindqvist, who conducted the research as a graduate student at Stanford University and is now a postdoctoral researcher. scientist in inorganic chemistry at Princeton University. “The unprecedented perovskite Au2+ could open up intriguing new possibilities.”
heavy electrons underneath
As an elemental metal, gold has long been valued for its relative rarity as well as its unique malleability and chemical inertness. This means it can easily be used to create jewelry and coins that do not react with chemicals in the environment and do not tarnish over time. Another important reason for its value is its namesake golden color; Perhaps no other metal in its pure form has such a pronounced, rich hue.
Karunadasa explained that the fundamental physics behind gold’s famous appearance also explains why Au2+ is so rare.
The main reason for this is the relativity effects proposed in Albert Einstein’s famous theory of relativity. “Einstein taught us that when objects move very fast and their speed approaches a significant fraction of the speed of light, objects become heavier,” Karunadasa said. said.
This phenomenon also applies to particles and has profound consequences for “large” heavy elements such as gold, whose atomic nuclei contain large numbers of protons. Together, these particles create a huge positive charge, causing the negatively charged electrons to spin around the nucleus at incredible speeds. As a result, the electrons become heavier and tightly surround the nucleus, reducing its charge and allowing the outer electrons to drift further than in typical metals. This rearrangement of electrons and energy levels causes gold to absorb blue light, thus appearing yellow to our eyes.
According to the theory of relativity, due to the electron configuration of gold, the atom naturally forms as Au1+ and Au3+, respectively, losing one or three electrons and discarding Au2+. (The “2+” sign indicates the net positive charge resulting from the loss of two negatively charged electrons, and the chemical symbol for gold, “Au,” comes from the Latin word “aurum,” meaning gold.)
Stanford researchers found that Au2+ can survive in the right molecular configuration. Lindqvist said he “stumbled upon” the new perovskite containing Au2+ while working on a larger project on magnetic semiconductors for use in electronic devices.
Lindqvist said he mixed the salt, cesium chloride and Au3+ chloride, with water and added hydrochloric acid to the solution “with a little bit of vitamin C.” In the following reaction, vitamin C (an acid) donates an electron to ordinary Au3+ (negatively charged) to form Au2+. Interestingly, Au2+ is stable in solid perovskite but not in solution.
“In the laboratory, using very simple materials, we can make this material at room temperature in about five minutes,” Lindqvist said. “The result is a dark green, almost black powder that is surprisingly heavy due to the gold it contains.”
Realizing that they might have reached a new chemical basis, so to speak, Lindqvist performed numerous tests on the perovskite, including spectroscopy and X-ray diffraction, to investigate how it absorbs light and characterizes its crystal structure. Stanford research groups in physics and chemistry, led by Yang Li Professor of Applied Physics and Photonics Science and Edward Solomon, the Monroe E. Spaght Professor of Chemistry and Professor of Photonics Science, have further contributed to the investigation of Au2+ behavior.
Experiments eventually confirmed the presence of Au2+ in perovskite, thus adding a chapter to the centuries-old history of chemistry and physics, which includes Linus Pauling, winner of the 1954 Nobel Prize in Chemistry and the 1954 Nobel Peace Prize. 1962. Early in his career he worked on gold perovskites containing the general forms Au1+ and Au3+. Coincidentally, Pauling later also studied the structure of vitamin C, one of the ingredients needed to make a stable perovskite containing the elusive Au2+.
“We love Linus Pauling’s connection to our work,” Karunadasa said. “Synthesis of this perovskite could make for a good story.”
Looking ahead, Karunadasa, Lindqvist and their colleagues plan to continue studying the new material and refine its chemical composition. Since the electrons in the perovskite jump from Au2+ to Au3+, it is hoped that Au2+ perovskite can be used in applications requiring magnetism and conductivity.
“We are excited to learn what Au2+ perovskite can do,” Karunadasa said. Source