Scientists have made surprising discoveries in battery technology
- May 15, 2024
- 0
Solid-state batteries store and release charge by pushing ions back and forth between two electrodes. From our usual point of view, ions flow in a smooth flow through
Solid-state batteries store and release charge by pushing ions back and forth between two electrodes. From our usual point of view, ions flow in a smooth flow through
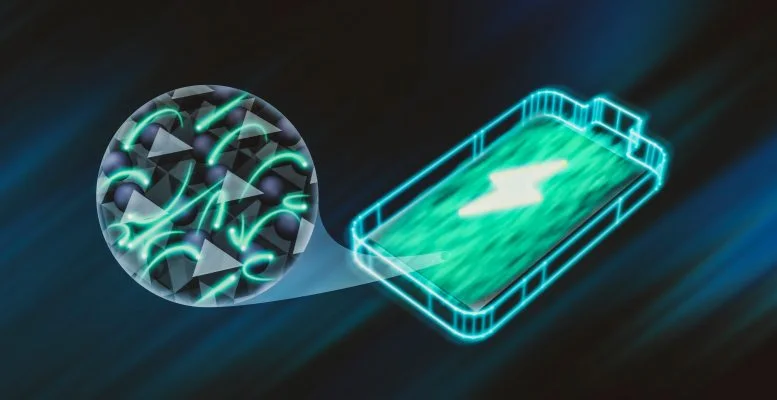
Solid-state batteries store and release charge by pushing ions back and forth between two electrodes. From our usual point of view, ions flow in a smooth flow through the solid electrolyte of the battery.
But when viewed at the atomic scale, this smooth flow is an illusion: Individual ions randomly jump from one open space to another in the electrolyte’s vast atomic lattice and are pushed toward the electrode by a constant voltage. These spikes are difficult to predict and difficult to trigger and detect.
Now, in the first study of its kind, researchers hit the bouncing ions with a pulse of laser light, delivering a jolt of voltage. Surprisingly, most of the ions briefly changed direction and returned to their previous positions before resuming their normal, more random movements. This was the first indication that the ions were somehow remembering where they had just been.
A research team from the Department of Energy’s SLAC National Accelerator Laboratory, Stanford University, Oxford University and Newcastle University announced their findings in this issue Nature For January 24th.
“You can think of the ions behaving like a mixture of corn starch and water,” said Andriy D. Poletaev, an Oxford postdoctoral researcher who helped run the experiment while he was a postdoctoral researcher at SLAC. If we gently push this cornstarch mixture, it will flow like liquid; but if we pierce it, it solidifies. The ions in the battery are like electronic cornstarch. By moving backwards, they counteract the strong shaking caused by the shock of the laser light.
The ions’ “fuzzy memory,” as Poletaev puts it, lasts only a few billionths of a second. But knowing its existence will help scientists predict what ions traveling for the first time will do next; This is an important point for the discovery and development of new materials.
For their experiments at the SLAC Laser Laboratory, the researchers used thin, transparent crystals of a solid electrolyte derived from a group of materials called beta-alumina. These materials were the first highly conductive electrolytes discovered. They contain small channels through which bounced ions can move quickly and have the advantage of being safer than liquid electrolytes. Beta alumina is used in solid-state batteries, sodium sulfur batteries, and electrochemical cells.
As the ions jumped through the beta-alumina channels, the researchers hit them with pulses of laser light lasting trillionths of a second and then measured the light returning from the electrolyte.
By varying the time between the laser pulse and the measurement, they were able to detect exactly how the speed and preferred direction of the ions changed within a few trillionths of a second after the laser pulse.
“A lot of weird and unusual things happen in the ion hopping process,” said SLAC and Stanford professor Aaron Lindenberg, a Stanford Institute for Materials Science and Energy (SIMES) researcher who led the study.
“When we apply a force that shakes the electrolyte, the ion does not react immediately as it does with most materials,” he said. “Ion may sit for a while, suddenly stand up, and then sit there again for a long time. You might have to wait a while, and then suddenly a huge change happens. So there’s an element of randomness in the process that makes these experiments difficult.”
Until now, the way the ions travel has been considered a classic “random walk”: they jostle, bump and gulp like a drunk man staggering down the sidewalk, but eventually reach a target in a way that may seem intentional, the researchers said. observer. Or consider a skunk releasing a foul-smelling spray into a room full of people; The molecules in the spray are randomly pushed and collided, but they reach your nose very quickly.
“This picture turns out to be wrong at the atomic scale” when it comes to skipping ions, Poletaev said, “but that is not the fault of the people who came to this conclusion. Researchers have been studying ion transport with macroscopic instruments for so long that they were not able to observe what we see in this study.”
He said the atomic-scale discoveries made here “will help bridge the gap between the atomic motions we can simulate on the computer and the macroscopic properties of the material that make our research so difficult.”
Source: Port Altele
As an experienced journalist and author, Mary has been reporting on the latest news and trends for over 5 years. With a passion for uncovering the stories behind the headlines, Mary has earned a reputation as a trusted voice in the world of journalism. Her writing style is insightful, engaging and thought-provoking, as she takes a deep dive into the most pressing issues of our time.