A new study using phosphorescence spectroscopy shows how small organic substances such as ethylene glycol disrupt the crystal structure of water ice, providing insights into the physical and chemical properties of ice.
Ice is believed to play a crucial role in the origin of life. One reason for this is that organic molecules can be incorporated into the interstices of the crystal lattice by the ordered water molecules, resulting in a concentration of organic compounds. However, current techniques for studying organic molecules in ice, such as Raman spectroscopy and infrared spectroscopy, are primarily limited to absorption-based spectroscopy methods, which limits the sensitivity of measurements.
A research team led by Professor Guoqing Zhang, Professor Shiyong Liu, Professor Xiaoguo Zhou and researcher Xuepeng Zhang from the University of Science and Technology of China (USTC) has developed a method to detect the microstructures of water and ice using organic phosphorescent probes and phosphorescence spectroscopy. Their work has been published Chemistry in question .
A new method for the analysis of organic ice molecules
The team proposed an emission-based method to study organic molecules in water ice. They used the hydration state of a phosphorescent probe, acridinium iodide (ADI), to indicate microstructural changes in water ice (i.e. crystalline or glassy). The microstructure of water ice can be largely determined by the trace amounts of water-soluble organic molecules. In particular, if water ice remains amorphous at low temperatures, the AD cation+ and anion i – The majority of the ADI probe will be separated by bound water molecules, exhibiting long-lived phosphorescence and a visible greenish-yellow glow. In regular crystalline ice, the ADI probe molecules will aggregate, resulting in short-lived red phosphorescence due to the influence of heavy iodine atoms.
Spectral analysis improves understanding of ice microstructures
The emission spectra revealed clear spectroscopic changes in the aqueous ADI solution upon addition of small molecules of ethylene glycol (EG) and monodisperse polymers of EG (PDI=1). Addition of trace amounts of EG (0.1%) results in the appearance of a fluorescence band around 480 nm, accompanied by a more intense phosphorescence band with well-separated vibronic advances at 555, 598, and 648 nm. Spectral results showed that addition of EG led to the transformation of ADI molecules in water ice from undissolved aggregates to dissolved ion states.
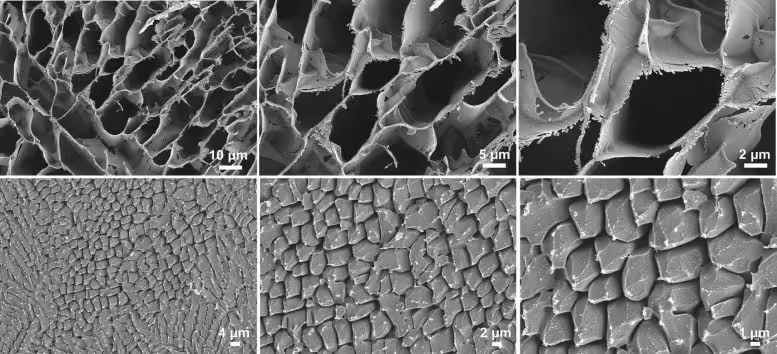
Validation of phosphorescence results using advanced imaging techniques
To confirm the phosphorescence spectroscopy findings, low-temperature scanning electron microscope (Cryo-SEM) images showed that the addition of trace amounts of EG to water ice containing ADI resulted in localized areas with porous microstructures. Meanwhile, low-temperature Raman scattering (LT-Raman) spectra confirmed that the addition of trace amounts of EG was sufficient to trigger the transition of the OH vibration of water ice from the low-frequency crystalline state to the high-frequency glassy state.
Implications for water-ice-organic interaction studies
This study found that adding trace amounts of small or large molecular organic matter to water can significantly suppress the crystalline order of water ice using more convenient and sensitive phosphorescence spectroscopy. In addition, phosphorescence spectroscopy can also detect morphological differences in the microstructure of water ice when trace organics of different structures and the same concentration are added to water, which is consistent with Raman spectroscopy and scanning electron microscopy, and provides a new technical tool for studying water ice. – Interactions of organic matter at a lower concentration and over a wider temperature range.