Scientists are one step closer to finding the brain’s REM sleep trigger
- October 27, 2024
- 0
Scientists have discovered the neural basis of REM sleep, a dream-like state of the brain in which the eyes are the only part of the body that is
Scientists have discovered the neural basis of REM sleep, a dream-like state of the brain in which the eyes are the only part of the body that is

Scientists have discovered the neural basis of REM sleep, a dream-like state of the brain in which the eyes are the only part of the body that is actively moving. When this circuit in the upper brainstem was triggered in mice, the researchers were able to put the animals into REM (rapid eye movement) sleep even though they were initially awake. If the results generalize to humans, we will be one step closer to understanding the biology of sleep and why it can go wrong.
This information could even help us better manipulate REM sleep in people with sleep apnea, narcolepsy, frequent disturbing nightmares, or REM sleep disorders that cause people to reenact their dreams with sounds like movement or sleep talking. REM sleep is shrouded in mystery, and research is complicated by the fact that scientists still don’t know where the REM sleep control center is located in the brain or whether such a center even exists.
For decades, some researchers have suspected that neurons in the mammalian brainstem play a critical role in the onset of REM. For example, if the brainstem in cats is cut off from the image, it is not possible to create correct REM sleep and the animals begin to reproduce their dreams. REM sleep may be similarly disrupted in people with known brainstem degeneration, similar to that seen in Parkinson’s disease.
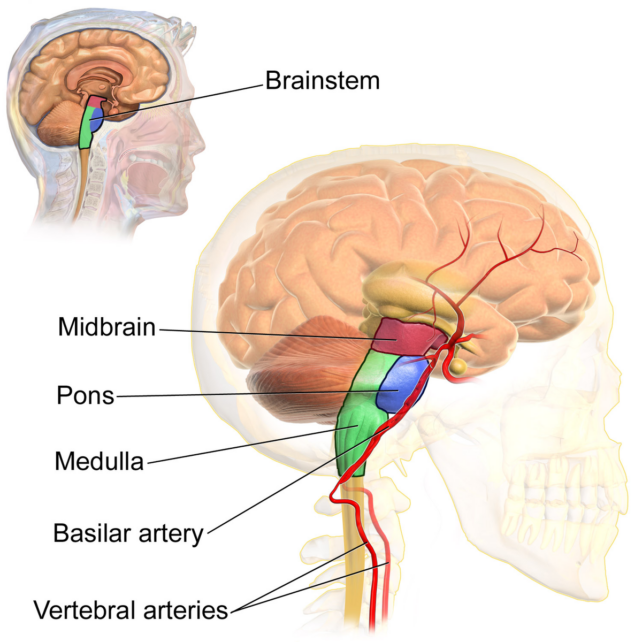
Over the years, further experiments in rodents found evidence that the pons, at the top of the brainstem, is the “control center” for the loss of normal muscle tension that limits movement during REM sleep. But because the neurons that support wakefulness in this part of the brain are mixed with those that support sleep, it has been difficult to pinpoint the pathways responsible for this important stage of sleep.
Neuroscientist Mitsuaki Kashiwagi of the University of Tsukuba and the University of Tokyo led a team in Japan and France to a cluster of REM-related neurons in the dorsal part of the pons. In mice, these neurons express corticotropin-releasing hormone-binding protein, so they are called Crhbp+ neurons.
These cells extend from the pons to the neurons in the medulla oblongata region, located just below the brainstem. Because they express nitric oxide synthase 1, they are called Nos1+ neurons. NOs1+ neurons then reconnect to Crhbp+ neurons as well as forebrain neurons.
Kasivaghi and colleagues say that this cycle from the pons to the medulla and back may function as the main circuit of REM sleep. When the team removed the bridge neurons from the positive feedback loop, the mice experienced reduced sleep and impaired muscle relaxation during REM sleep.
However, when pontine neurons projecting to the medulla were activated, mice entered REM sleep more quickly, and the number and duration of REM sleep episodes during sleep increased at the expense of wakefulness. Nos1+ neurons in the medulla contributed strongly to REMS by projecting to various regions involved in REM activity.
In fact, activation of these neurons in mice caused a direct transition from wakefulness to REM sleep. Even when non-REM sleep first occurred, it was very short and the mice entered REM sleep more quickly. Neurons projecting into the forebrain appear to inhibit wakefulness.
Narcolepsy patients are known to go directly from wakefulness to REM sleep, but this jump is very unusual.
“Having identified Crhbp as a marker for sleep-regulating neurons, we tested whether these neurons were affected in Parkinson’s patients with REMS behavior disorder,” the authors explain.
The team found, of course, that Crhbp-immunoreactive neurons were significantly reduced in this cohort; this “provides insight into the mechanisms underlying the sleep deficits that characterize this disease.” In a mouse model of Parkinson’s disease, researchers showed that activation of Crhbp+ neurons in the pons could reverse the observed sleep disturbances.
Kasivaghi and colleagues say the next step is to record the activity of these neurons at single-cell resolution to understand what they actually do and why. The study was published on: Cell.
Source: Port Altele
As an experienced journalist and author, Mary has been reporting on the latest news and trends for over 5 years. With a passion for uncovering the stories behind the headlines, Mary has earned a reputation as a trusted voice in the world of journalism. Her writing style is insightful, engaging and thought-provoking, as she takes a deep dive into the most pressing issues of our time.