A team from the Massachusetts Institute of Technology (MIT) has confirmed the benefits of removing carbon dioxide from seawater rather than the atmosphere. Research data is presented in the article in the journal. Energy and Environmental Science. A practical demonstration of the proposed technology will take place over the next two years. If all goes well, a new type of business will emerge in the world – CO2 extraction with seas and oceans commercially and without subsidies.
According to the IEA (International Energy Agency) for 2022, the most effective technologies for capturing carbon dioxide from the atmosphere require approximately 6.6 GJ of energy per tonne of CO captured, or 1.83 MWh.2. A significant portion of this energy is used to maintain the operating temperatures of the absorbers or the operation of compressors that compress the air to effective pressure values.
By some estimates, the cost of capturing one tonne of CO by 2030 2 It will be between $300 and $1000. Today, the highest carbon tax is imposed on industrialists in Uruguay: $137 per ton. This amount does not cover and does not cover the cost of CO removal for a long time.2 MIT is sure that everything could be different with the atmosphere, but with seawater.
Extracting carbon dioxide from seawater is more profitable only because its concentration there is 100 times higher than in air. Oceans and seas are natural absorbers of CO.2. Seawater is claimed to absorb 30-40% of humanity’s annual carbon emissions. Previously, methods were proposed to remove carbon dioxide from water, but these required expensive membranes for filtration or a constant supply of chemical reagents.
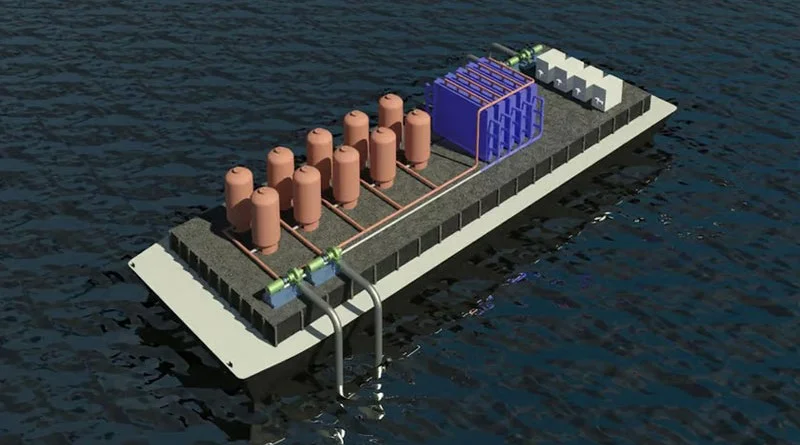
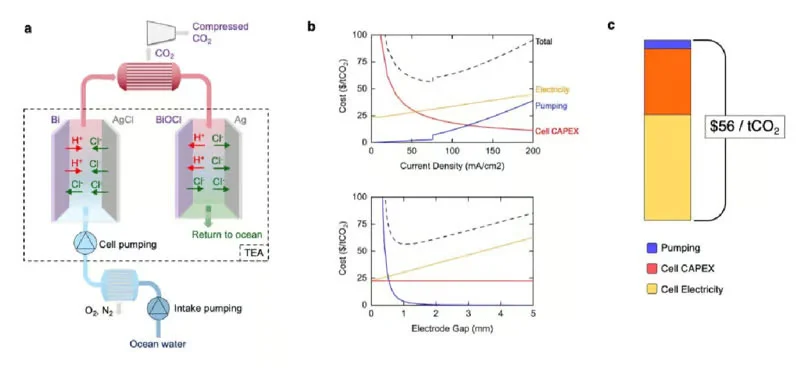
In the system proposed by MIT scientists, seawater passes through two chambers. Current passing through the electrodes in the first chamber saturates the liquid with protons and acidifies it, converting dissolved inorganic bicarbonates into carbon dioxide. In the vacuum chamber, the liquid is degassed and carbon dioxide is removed. In the second chamber, the reverse polarity at the electrodes causes the protons to settle on the electrical contacts, which alkalizes the water and then spills into the ocean.
When the electrodes in the first chamber are depleted and saturated with protons in the second chamber, the polarity of the voltage applied to the chambers changes and water can be pumped in reverse order with the same result – it is taken up by the second chamber and expelled. from the first – with the same effect of CO extraction 2 . Then the process is repeated in reverse order. Ultimately this leads to contamination of the electrodes with sedimentary minerals, but this is a solvable problem.
Ultimately, the proposed process allows alkaline-balanced water to be returned to the ocean, which will be good for the environment. The ocean is acidifying – this has led to the death of coral reefs and marine life, for example.
Scientists do not hide that they will have to work hard to implement the project. There are no ready projects to present, but these are all solvable tasks and there is nothing to fear from them. Ultimately, scientists hope to reduce the cost of removing carbon dioxide from seawater to $56 per tonne. This will be an impetus for the commercialization of this direction.








:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/33SLWRSASJCBTKODQFRIOP5MXU.jpg)



