Carbon dioxide (CO2), is the most common greenhouse gas causing climate change, but it was present on Earth long before humans began releasing it into the atmosphere at unprecedented levels. Thus, some of the planet’s oldest organisms have evolved to use and use this gas, which would otherwise be harmful to humans and the planet.
One of these processes, called the Wood-Lyungdahl pathway, takes place only in the absence of oxygen and is considered the most efficient pathway for carbon fixation in nature. But exactly how the path goes from one step to the next remains unclear.
Now, scientists at the Stanford Synchrotron Radiation Facility (SSRL) at the SLAC National Accelerator Laboratory at the University of Michigan, Northwestern University and Carnegie Mellon University have discovered the previously unknown inner workings of the Wood-Ljungdahl pathway.
The findings, published Journal of the American Chemical Society The past month not only shed light on one of the oldest chemical reactions on Earth, but could also lead to improved carbon capture methods to mitigate climate change.
“Before this study, we knew that the Wood-Ljungdahl pathway to produce carbon for use by organisms begins with carbon dioxide,” said Macon Abernathy, SSRL research fellow and co-author of the study. “Then it converts the CO2 on carbon monoxide and a methyl group, and some chemical magic turns them into a form of carbon the body can use.”
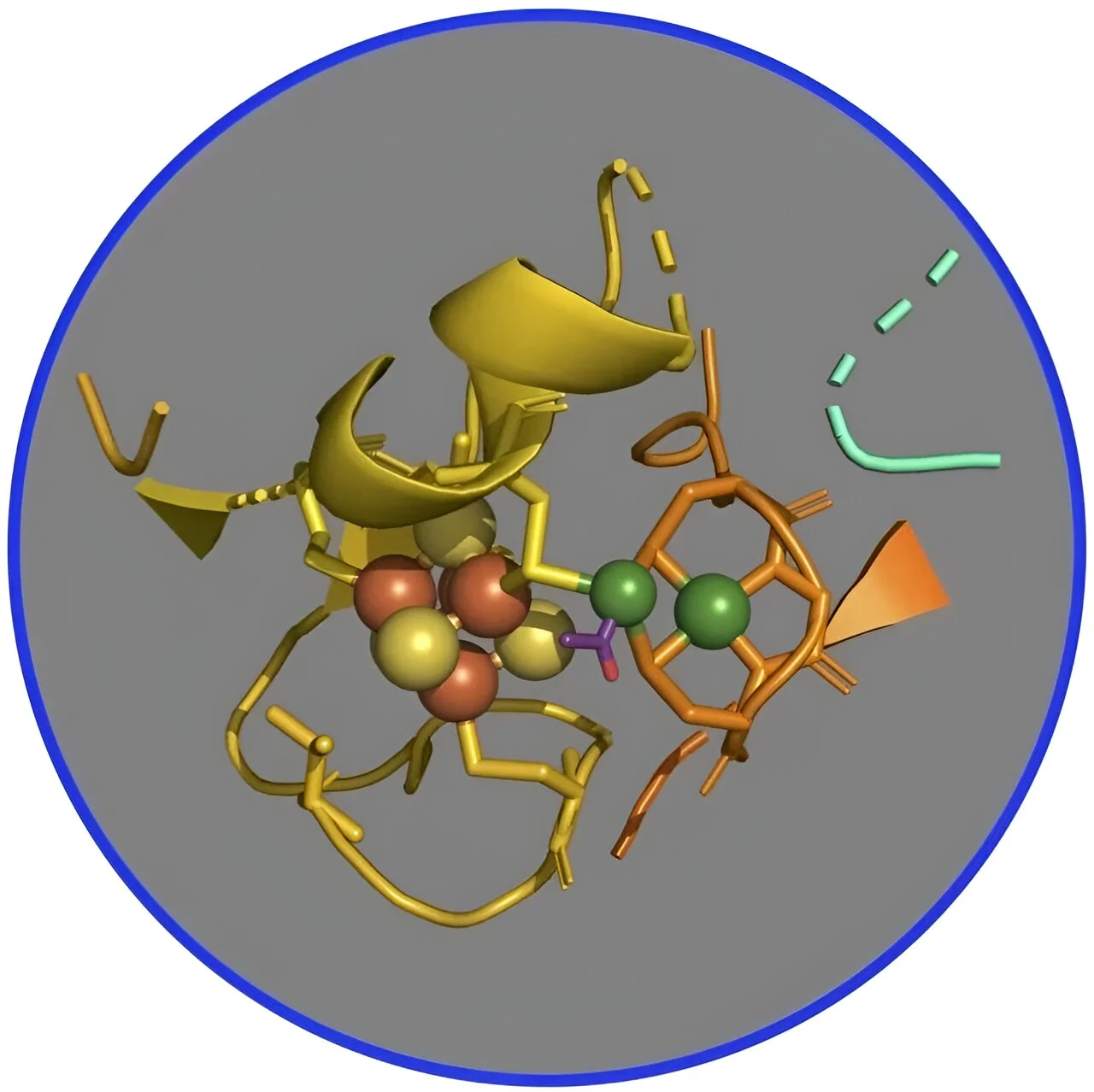
For many years, scientists have suggested that this pathway goes through a series of nickel-based organometallic intermediates that form metal-carbon bonds. Specifically, the researchers focused on two nickel-iron-sulfur protein complexes, called CO dehydrogenase and acetyl-CoA synthase (CODH/ACS), which are the main enzymes that catalyze the conversion of carbon dioxide into energy and structural carbon. cell walls and proteins.
But confirming this hypothesis has proven difficult because the enzyme complex must be purified in an oxygen-deficient atmosphere, such as on early Earth 4 billion years ago, when these proteins and this pathway arose. In addition, intermediates are often unstable and the reaction can quickly become ineffective. Also, the presence of other nickel and iron atoms in CODH hinders the study of ACS, which is the aim of this study.
To overcome these problems, the researchers designed a more active version of the ACS-only protein that does not contain CODH and used X-rays in SSRL to understand its metals and how they work within the enzyme. The team used X-ray spectroscopy, a technique in which the scientists study the interference of light that is absorbed, released by metals, and then returned to the metals in the complex (here, ACS) to determine changes in chemical bonds during reactions. .
In a word, the scientists confirmed their long-held hypothesis.
“We discovered that a very complex part of organometallic chemistry goes on, where a nickel site in the enzyme does all the interesting things,” said Ritimukta Sarangi, a senior scientist at SSRL and corresponding author of the study.
Steve Ragsdale, a professor at the University of Michigan and author of the answer, the team learned that although the enzyme contains two nickel clusters each attached to four iron and sulfur atoms, the reaction always occurs at a particular nickel in the cluster. . for research. “The carbons like carbon monoxide, the methyl group, and the acetyl group all bind most closely to nickel in iron and sulfur, and it’s pretty obvious that they don’t bind to any other metals.”
The researchers also noticed that the nickel-containing protein undergoes major changes in its structure in each of the intermediate states, Ragsdale said. “This was something that wasn’t really part of our original hypothesis. We just thought the basic chemistry was based on nickel. But then we see all the other changes that happen in the protein, which was a little surprising.”
Abernathy said that even though the researchers had a clear idea of how the reaction works, it was still impressive to see him in action.
“It’s a very fine tuning of nature to bring out this elegant system that does this catalysis,” Sarangi said. “I love this and our ability to use X-ray spectroscopy, an extremely powerful tool to understand what’s going on in nature. SSRL’s Structural Molecular Biology Resource has a world-leading biological X-ray spectroscopy program that enables the study of such complex biological processes.”
While praising the natural beauty of the Wood-Ljungdahl road, Ragsdale expressed hope that research to better understand and perhaps improve these natural processes could lead to ways to mitigate climate change and improve carbon capture for chemical feedstock production. and fuel. “I think we need to understand the basic biochemistry behind the process before we can make progress in improving some of these pathways that exist in nature,” he said. Source