Old stars may produce elements with more than 260 protons
- December 23, 2023
- 0
The first stars of the universe were monstrous creatures. These particles, composed solely of hydrogen and helium, can have masses up to 300 times greater than the Sun.
The first stars of the universe were monstrous creatures. These particles, composed solely of hydrogen and helium, can have masses up to 300 times greater than the Sun.
The first stars of the universe were monstrous creatures. These particles, composed solely of hydrogen and helium, can have masses up to 300 times greater than the Sun. The first heavy elements were formed in them and were thrown into space at the end of their short lives. They were the seeds of all the stars and planets we see today. A new study has been published ScienceThis suggests that ancient ancestors created more than just natural elements.
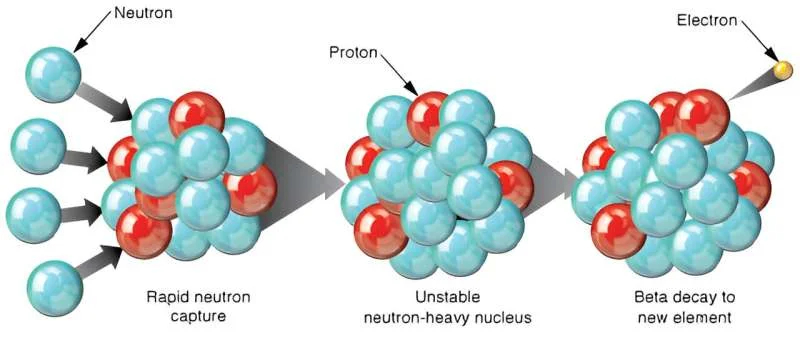
Except for a few traces of hydrogen, helium, and other light elements, all the atoms we see around us were created by astrophysical processes such as supernovae, neutron star collisions, and high-energy particle collisions. Together, they created heavier elements, up to uranium-238, the heaviest naturally occurring element. Uranium is formed by the collisions of supernovae and neutron stars through the so-called r-process, in which neutrons are rapidly captured by atomic nuclei and become a heavier element. The R-process is complex, and we still don’t quite understand how it forms or what its upper mass limit might be. But this new study suggests that the r-process in the oldest stars may have created much heavier elements with atomic masses greater than 260.
The team looked at 42 stars in the Milky Way whose elemental compositions were well studied. Instead of just looking for the presence of heavier elements, they looked at the relative abundance of the elements in all stars. They found that the abundances of some elements, such as silver and rhodium, were inconsistent with abundances predicted from the known process of r-nucleosynthesis. Evidence suggests that these elements are remnants of the decay of much heavier nuclei with more than 260 atomic mass units.
In addition to the r-process of fast neutron capture, there are two other ways to form heavy atomic nuclei: the p-process, in which neutron-rich nuclei capture protons, and the s-process, in which an embryonic nucleus can capture a neutron. . However, none of these can provide the rapid mass accumulation required for elements other than uranium. And only in hypermassive stars of the first generation, r-process nucleosynthesis was able to create such elements.
Therefore, the study suggests that the r-process may have created elements far beyond uranium, and possibly in the universe’s first stars as well. If there wasn’t an island of stability for some of these superheavy elements, they would have long ago decayed into the natural elements we see today. But the fact that they once existed will help scientists better understand the r-process and its limits.
Source: Port Altele
As an experienced journalist and author, Mary has been reporting on the latest news and trends for over 5 years. With a passion for uncovering the stories behind the headlines, Mary has earned a reputation as a trusted voice in the world of journalism. Her writing style is insightful, engaging and thought-provoking, as she takes a deep dive into the most pressing issues of our time.