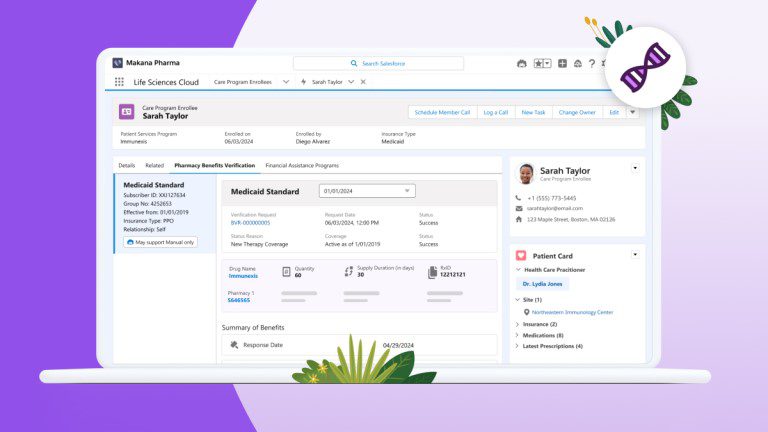
Salesforce announces the general availability of Life Sciences Cloud, a platform aimed at pharmaceutical and medical device companies that helps personalize interactions with patients and healthcare professionals.
Salesforce introduces the Life Sciences Cloud. This new platform is primarily designed to simplify clinical trials.
According to Salesforce, one of the biggest challenges in clinical trials is recruiting suitable candidates, which can take up to a third of the time of a study. The new platform accelerates this process through AI-driven participant recruitment and enrollment. Research coordinators can quickly screen patients based on pre-selection criteria and assign them to suitable studies. This reduces the manual screening period and increases efficiency, according to Salesforce.
The platform also offers the ability to publish patient portals. These portals make it easy for eligible patients to discover clinical trials and express their interest. In addition, coordinators can customize electronic consent and assessment forms to streamline the enrollment process.
Data cloud
The Life Sciences Cloud works with a Unified data platformbased on the Data Cloud and MuleSoft for Life Sciences. This platform collects and harmonizes data from various sources such as electronic health records, claims databases and social platforms. This enables organizations to create patient profiles, which in turn help generate target groups for specific therapies or clinical trials.
The platform also provides tools to manage patient programs during clinical trials, allowing teams to develop strategies to remind patients to take their medications and analyze the effectiveness of those strategies during the campaign.
With Life Sciences Cloud, Salesforce aims to bring together its existing products and expertise tailored to a demanding industry. This gives you a ready-made suite tailored to relevant and unique problems. Life Sciences Cloud is now generally available, with several features to be released in 2024 and 2025.