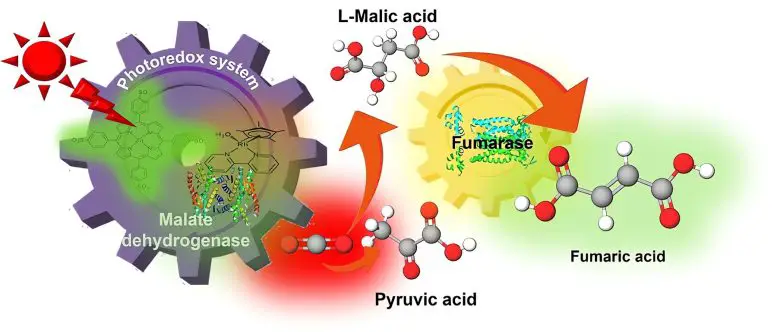
Synthesis of fumaric acid by a new artificial photosynthesis method using sunlight. In recent years, environmental problems caused by global warming due to greenhouse gases such as CO have become more prominent.2. CO during natural photosynthesis2 is not directly restored, but binds to organic compounds that are converted into glucose or starch. By mimicking this, artificial photosynthesis can reduce CO emissions2 by combining them into organic compounds for use as raw materials that can be converted into durable forms such as plastics.
A research group led by Professor Yutaka Amao from the Artificial Photosynthesis Research Center and PhD student Mika Takeuchi from the Osaka Metropolitan University Institute of Science has succeeded in synthesizing fumaric acid from CO2.2, raw materials for the manufacture of plastics used for the first time – by sunlight. Their findings have been published Sustainable Energy and Fuels.
Normally fumaric acid is synthesized from petroleum for use as a raw material in the production of biodegradable plastics such as polybutylene succinate, but this discovery shows that fumaric acid can be synthesized from CO.2 and compounds derived from biomass using renewable solar energy.
“For the practical application of artificial photosynthesis, this research has succeeded in using visible light – renewable energy – as an energy source,” said Professor Amao. “In the future, we plan to collect gas CO2 and use it to synthesize fumaric acid directly through artificial photosynthesis.”