We used to think that the atom was the smallest building block of matter, but today we know that the atom; It consists of three subatomic particles called protons, electrons and neutrons. The nucleus, the center of the atom, consists of positive protons and uncharged neutrons. The negatively charged electron is located around the nucleus. We understand this So where is the atomic number in this? If you say, it’s actually right in the middle.
What we call the atomic number is actually the number of protons of an element and thus the number of electrons equal to it in the neutral state. It is the atomic number that makes matter matter. The atomic number next to each element in the periodic table of chemical elements is unique and cannot be changed. If it is changed, it will already turn into another element. What is the bride’s best atomic number, how to find it Let’s take a closer look at that.
Let’s start with a basic definition; What is Atomic Number?
The atomic number, represented by the symbol Z, refers to the number of protons in the nucleus of each chemical element. Since the number of electrons and protons in an atom in neutral state are equal, we can say that the number of protons, number of electrons and atomic number of a chemical element in neutral state are the same. Of course, these are ordinary elements, there may be different situations.
So, what exactly does the atomic number mean?
We opened the periodic table, we found the element iron, next to it is written 26; This means that the atomic number of the element iron is 26. In other words, there are 26 protons in an iron atom. For example, the atomic number of the element nitrogen is 7, that is, there are 7 protons in the nitrogen atom.
The atomic number, that is, the number of protons, is the basic element that makes that atom that atom. Let’s say we change the number of protons, another element emerges. Let’s say we change the number of electrons, we get an ion. Let’s say we change the number of neutrons, an isotope is created. In other words, an element’s atomic number is the most basic and unique characteristic by which we can identify that element.
So how do you find the atomic number?
We’d like to say you can find it at home, but the human eye hasn’t evolved to see subatomic particles yet. That’s the atomic number take the atom you want to know which element it is, You put it in a device called an atomic microscope or a quantum microscope and you start counting the protons.
Let’s say the atom you’re examining under a microscope has 26 protons, so you have the element iron. Let’s say the atom has 33 protons, so you have arsenic. It is possible to multiply such examples. In summary, the atomic number is found by measuring the number of protons in an atom.
What is the atomic number equal to?
The atomic number is equal to the number of protons and the number of protons is equal to the number of electrons in the neutral state. So how many neutrons does this atom have? Now let’s do the math; Let’s say you have calcium in your hand, the atomic number of calcium is 20, that is, the number of protons is 20. It is known that the relative atomic mass of calcium is 40. Now let’s subtract the atomic number from the relative atomic mass. ie 40 – 20 = 20. There are 20 neutrons in a calcium atom. You can apply this process to all chemical elements.
How does the atomic number increase?
In fact, there is no such thing as an increase in atomic number because All known elements have an atomic number and this number is fixed. As the number of protons in the atom increases, so does the atomic number. But this is an increase that we learn by counting. Of course, if you want to create an atomic reaction, change jobs.
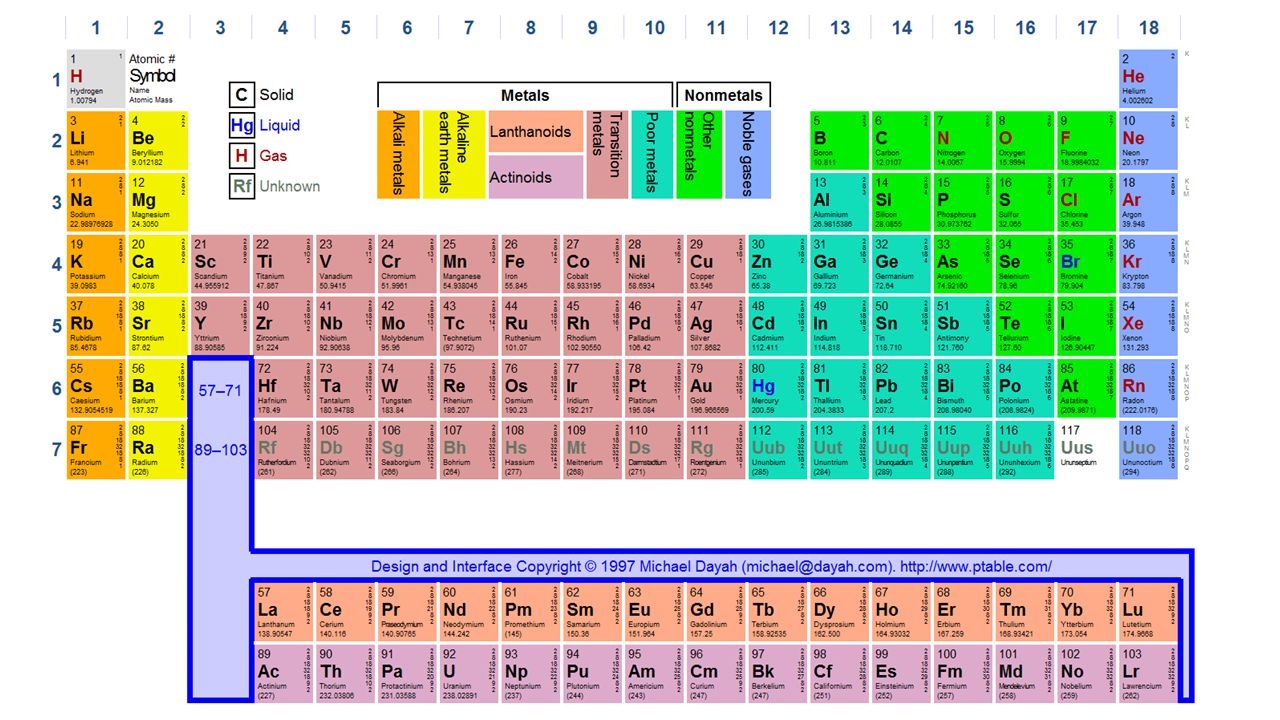
All chemical elements in the periodic table according to their atomic numbers:
| atomic number |
symbol |
Element |
Relative atomic mass |
| An |
H |
Hydrogen |
1.0079 |
| 2 |
He |
Helium |
4.0026 |
| 3 |
li |
Lithium |
6,941 |
| 4 |
are |
Beryllium |
9.0122
|
| 5 |
B |
drill |
10,811
|
| 6 |
C |
Carbon |
12.0107 |
| 7 |
N |
Nitrogen |
14.0067 |
| 8 |
HE |
Oxygen |
15.9984 |
| 9 |
F |
Fluorine |
18.9984 |
| 10 |
What |
Neon |
20.1797 |
| 11th |
after |
Sodium |
22.9897 |
| 12 |
mg |
Magnesium |
24,305 |
| 13 |
To get |
aluminium |
26.9815 |
| 14 |
Si |
Silicon |
28.0855 |
| 15 |
P |
Phosphorus |
30.9738 |
| 16 |
S |
Sulfur |
32,065 |
| 17 |
cl |
Chlorine |
35,453 |
| 18 |
Ar |
Argon |
39,948 |
| 19 |
K |
Potassium |
39.0983 |
| 20 |
Approx |
Calcium |
40,078 |
| 21 |
sc |
scandium |
44.9559 |
| 22 |
Ti |
Titanium |
47,867 |
| 23 |
v |
i’m vanda |
50.9415 |
| 24 |
cr |
chrome |
51.9961 |
| 25 |
mn |
manganese |
54,938 |
| 26 |
Fe |
Iron |
55,845 |
| 27 |
co |
Cobalt |
58.9332 |
| 28 |
Ni |
Nickel |
58.6934 |
| 29 |
C |
Buyer |
63,546 |
| 30 |
Zn |
Zinc |
65.39 |
| 31 |
Go |
Gallium |
69,723 |
| 32 |
ge |
Germanium |
72.64 |
| 33 |
ace |
Arsenic |
74.9216 |
| 34 |
Like this |
Selenium |
78.96 |
| 35 |
bro |
Bromine |
79,904 |
| 36 |
kr |
Krypton |
83.8 |
| 37 |
rb |
Rubidium |
85.4678 |
| 38 |
sr. |
strontium |
87.62 |
| 39 |
Y |
Yttrium |
88.9059 |
| 40 |
Sr |
Zirconium |
91,224 |
| 41 |
note |
Niobium |
92.9064 |
| 42 |
before Christ |
Molybdenum |
95.94 |
| 43 |
tc |
technetium |
98 |
| 44 |
ru |
ruthenium |
101.07 |
| 45 |
Rh |
Rhodium |
102.9055 |
| 46 |
pd |
palladium |
106.42 |
| 47 |
Network |
Silver |
107.8682 |
| 48 |
CD |
Cadmium |
112,411 |
| 49 |
By |
Indium |
114,818 |
| 50 |
Mr. |
look |
118.71 |
| 51 |
z |
Antimony |
121.76 |
| 52 |
At |
Tellurium |
127.6 |
| 53 |
i |
Iodine |
126.9045 |
| 54 |
xe |
Xenon |
131,293 |
| 55 |
cs |
cesium |
132.9055 |
| 56 |
ba |
Barium |
137,327 |
| 57 |
la |
lanthanum |
138.9055 |
| 58 |
ce |
Cerium |
140.116 |
| 59 |
pr |
Praseodymium |
140.9077 |
| 60 |
Nd |
neodymium |
144.24 |
| 61 |
P.M |
promethium |
145 |
| 62 |
sm |
Samarium |
150.36 |
| 63 |
EU |
europium |
151,964 |
| 64 |
Gd |
Gadolinium |
157.25 |
| 65 |
phone |
Terbium |
158.9253 |
| 66 |
Tue |
dysprosium |
162.5 |
| 67 |
hi |
Holmium |
164.9303 |
| 68 |
private |
erbium |
167,259 |
| 69 |
tm |
Thulium |
168.9342 |
| 70 |
Yb |
ytterbium |
173.04 |
| 71 |
lu |
Lutetium |
174,967 |
| 72 |
HF |
Hafnium |
178.49 |
| 73 |
ta |
tantalum |
180.9479 |
| 74 |
W |
Tungsten |
183.84 |
| 75 |
Regarding |
Rhenium |
186.207 |
| 76 |
ox |
Osmium |
190.23 |
| 77 |
ir |
Iridium |
192.217 |
| 78 |
pt |
Platinum |
195,078 |
| 79 |
ouch |
Gold |
196.9665 |
| 80 |
hg |
Mercury |
200.59 |
| 81 |
TL |
Thallium |
204.3833 |
| 82 |
pb |
Ball |
207.2 |
| 83 |
A |
Bismuth |
208.9804 |
| 84 |
po |
Polonium |
209 |
| 85 |
Horse |
Astatine |
210 |
| 86 |
rn |
Radon |
222 |
| 87 |
Fri. |
francium |
223 |
| 88 |
ra |
Radium |
226 |
| 89 |
Hungry |
actinium |
227 |
| 90 |
E |
Thorium |
232.0381 |
| 91 |
father |
protactinium |
231.0359 |
| 92 |
YOU |
Uranium |
238.0289 |
| 93 |
np |
neptunium |
237 |
| 94 |
pu |
Plutonium |
244 |
| 95 |
am |
americium |
243 |
| 96 |
cm |
curium |
247 |
| 97 |
bk |
Berkelium |
247 |
| 98 |
cf |
California |
251 |
| 99 |
Husband |
einsteinium |
252 |
| hundred |
fm |
fermium |
257 |
| 101 |
md |
Mendelium |
258 |
| 102 |
No. |
Nobelium |
259 |
| 103 |
Lr |
lawrenium |
262 |
| 104 |
rf |
Rutherfordium |
261 |
| 105 |
db |
dubnium |
262 |
| 106 |
sg |
seaborgium |
266 |
| 107 |
bra |
bohrium |
264 |
| 108 |
hs |
Hassium |
277 |
| 109 |
mountain |
meitnerium |
268 |
Determined by the number of protons that chemical elements have What is atomic number and how to find it We’ve listed all the chemical elements in the periodic table according to their atomic numbers by answering curious questions like: