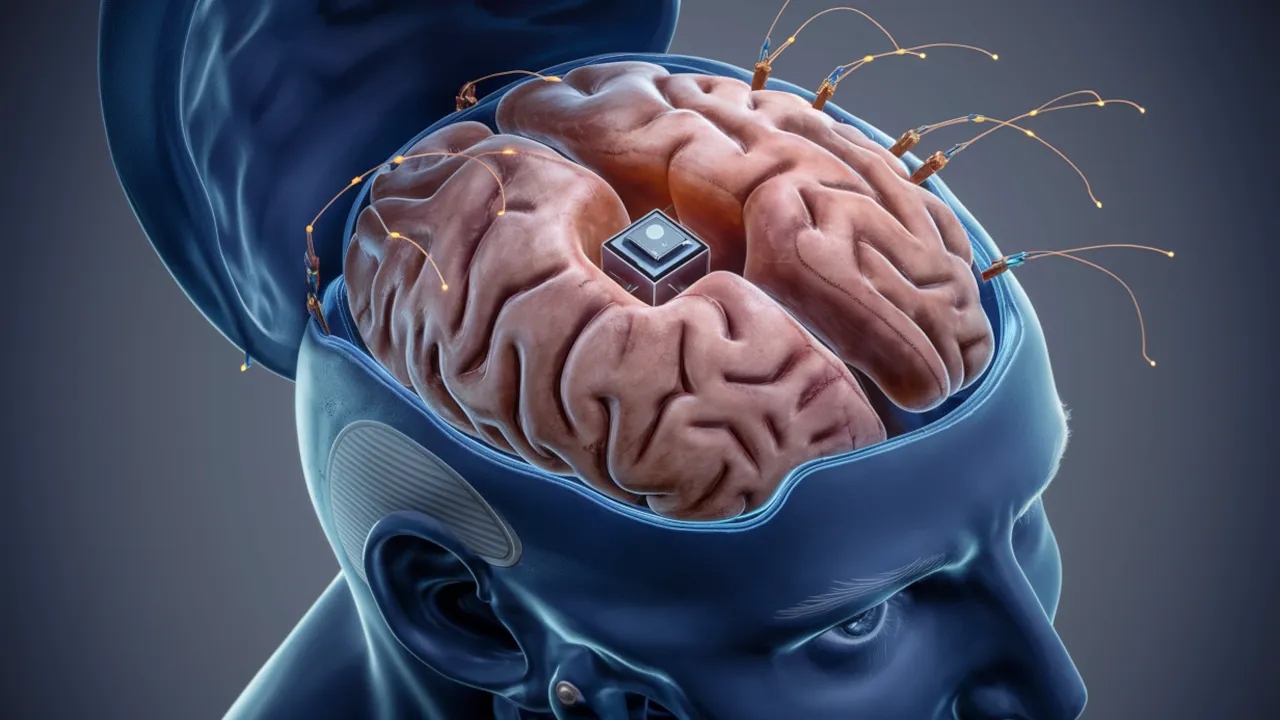
Elon Musk’s Neuralink brain chip initiative has begun its first human experiments in recent months. With surgery performed in January, 29-year-old paraplegic Noland Arbaugh became the first person to have a chip inserted into his brain. The later released videos showed Arbaugh being able to do many things with just the power of his mind, from playing games to sharing on social media.
Now a development has taken place that Neuralink will be happy with. US Food and Drug Administration (FDA), The company is officially allowed to continue testing. approved. This news emerged in a report published by the Wall Street Journal (WSJ).
A second person receives a Neuralink chip
The approval from the US health regulator FDA comes after the company’s initial tests will solve the problems came after he declared it. The company recently announced that it had encountered some problems, stating that there were differences in the positions of the wires in the chip installed in Arbaugh. According to the report, it wants to solve these problems by anchoring it more deeply.
It is not yet clear who Neuralink’s second patient will be. from the company Second attempt in June is expected to begin. Let us also add that the aim is to place the implant in a total of ten people by the end of the year. According to WSJ so far More than 1000 paralyzed people requested for testing.
The brain-computer interface system developed by Neuralink aims to control devices such as computers through thoughts and thus help paralyzed patients.
Follow Webtekno on Threads and don’t miss the news